Describe the Two Categories Used to Classify Physical Changes
These physical properties can be used to classify substances. Describe two ways you can separate a mixture using a physical property.
Physical Change Chemical Change Rusting Of Iron And Crystalization
Define and state whether dissolving can be reversed.

. Chemistry End of Chapter Exercises. Classify the six underlined properties in the following paragraph as chemical or physical. When a physical change happens the objects shape and size are changed.
A physical change does not result in the production of. A chemical substance is composed of one type of atom or molecule. Density ρ Mass Volume.
A heterogeneous mixture is obviously a mixture such as dirt. Differences in physical properties such as. Any sample of sucrose table sugar consists of 421 carbon 65 hydrogen and 514 oxygen by mass.
Particle size phase solubility boiling point. Reversible which can be undone. The Holdridge Life Zone Classification scheme uses latitude altitude and humidity to identify a regions climate see the diagram at right.
Describe the two categories used to classify physical changes. Click for a larger view. For example in the case of physical state a substance can be either a solid a.
Changes in state or phase melting freezing vaporization condensation sublimation are physical changes. Mixtures are physically combined structures that can be separated into their original components. A homogeneous mixture behaves like a single substance such as saltwater.
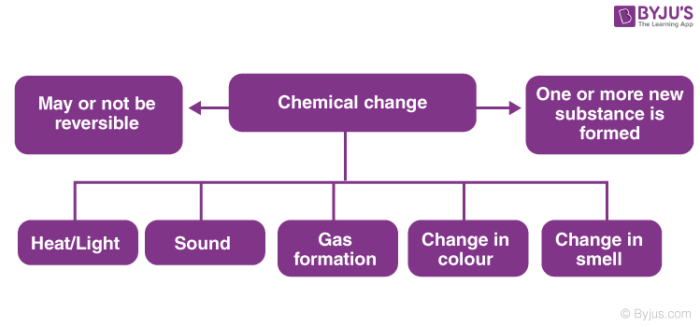
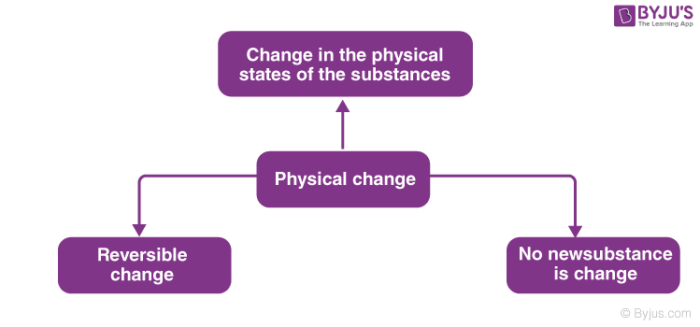
However in a chemical change the kind of matter changes and at least one new substance with new properties is formed. A physical change does not produce a new substance although the starting and ending materials may look very different from each other. Explain the term phase as it relates to homogeneous and heterogeneous mixtures.
Chemical changes involve the rearrangement of molecules or atoms and result in the pro-duction of one or more new substances. In a physical change the appearance or form of the matter changes but the kind of matter in the substance does not. A physical change is defined as a change in the state or energy of matter.
Fluorine is a pale yellow gas that reacts with most substancesThe free element melts at 220 C and boils at 188 CFinely divided metals burn in fluorine with a bright flameNineteen grams of fluorine will react with 10 gram of hydrogen. Pure substances and mixtures. Pure substances are further broken down into elements and compounds.
Physical changes are concerned with energy and states of matter. Physical properties describe the existence of matter and chemical properties describe how substances change into other substances. Intensive properties depend on the nature of the substance.
Physical properties describe the existence of matter and chemical properties describe how substances change into other substances. Physical changes are either reversible or irreversible. Irreversible changes can not be undone.
Describe the two categories used to classify physical changes. Shape form or state of matter in which the matters identity stays the same. And irreversible which is permanent.
A pure substance has a constant composition. A chemical change is defined as a change in the composition and properties of a substance. A heterogeneous mixture is obviously a mixture such as dirt.
Mass length pressure etc. Extensive properties such as mass and volume depend on the amount of matter being measured. The distinction between physical and chemical change is not clear cut.
Make a flow chart. A phase is any part of a sample with a uniform composition. Physical properties can be measured without changing a substances chemical identity.
Describe the two categories used to classify properties of matter. Reversible changes can be undone. A homogeneous mixture behaves like a single substance such as saltwater.
Intensive properties such as density and color do not depend on the amount of the substance present. The extensive properties are properties that you can measure ie. Or a physical change.
As classifications are based on plotting just three physical parametersthe straightforward system has become popular in modeling climate change impacts. All specimens of a pure substance have exactly the same makeup and properties. Properties that depend on the extent of the substance.
Two broad categories are mixtures and pure substances. There is one phase in a homogeneous mixture and two or more in a heterogeneous mixture. Sometimes these are the ratio of 2 extensive properties.
Matter can be classified into several categories. Matter can be broken down into two categories.
Physical Change Chemical Change Rusting Of Iron And Crystalization

Physical Change Chemical Change Rusting Of Iron And Crystalization

Physical And Chemical Changes Write And Draw Worksheet Teaching Chemistry Chemistry Worksheets Chemistry Classroom

Teaching With Elly Thorsen What S Growin In My Classroom April 2015 Secondary Physical Science Middle School Chemical And Physical Changes Chemical Changes
No comments for "Describe the Two Categories Used to Classify Physical Changes"
Post a Comment